My research in Osteoarthritis (OA) started in 2004 as a collaboration between the Image group at the IT University of Copenhagen and the Center for Clinical and Basic Research in Ballerup. Since then, the project has moved with me, being located at Nordic Bioscience and then Synarc Imaging Technologies. The project is now based at Biomediq, but we still collaborate with CCBR, Nordic Bioscience and the Image group at University of Copenhagen. We are also a partner in the D-BOARD consortium, an EU FP7 project. For D-BOARD info, visit d-board.eu.
The description below is quite outdated, but it nicely reflects the original focus on the project.
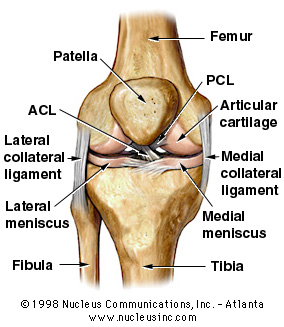
The focus of the project is osteo-arthritis (in Danish: "slid-gigt") and quantification of the disease progression. In particular, this means measuring and modelling the articular cartilage ("led-brusk") in the knee. The articular cartilage is located at the bottom surface of the Femur ("lårbens-knoglen") and on the top part of the Tibia ("skinnebens-knoglen") and serves as a smooth shock absorber between the bones. A main effect of OA (osteo-arthritis) is wear-down of the articular cartilage which is very painful since the bones then start scraping directly against each other.

|
The primary end goal of the project is to be able to accurately measure the effect of drugs on the development of the knee cartilage. This will enable the pharmaceutical companies to develop drugs that can effectively help people suffering from OA.
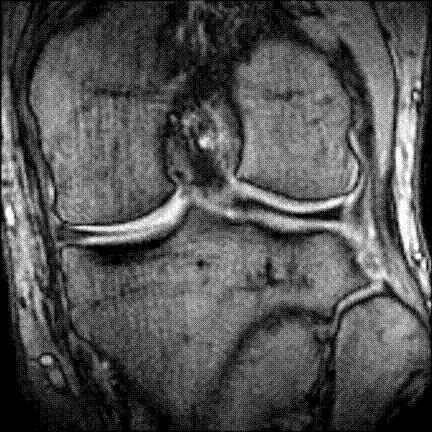
Presently, the clinical practice is to quantify the disease progression by measuring the joint gap from an X-ray ("røntgen") image of the knee. This is a seamingly hopeless task since cartilage is invisible in x-ray images. Therefore, the cartilage is estimated indirectly through a measurement of the distance between the Tibia and Femur bones. This is problematic since the cartilage is not necessarily touching (depending on the pose of the knee, see figure 2) and therefore a part of the gap is filled with joint fluid ("ledvæske") or the meniscus ("menisken"). However, another key effect of OA is the deformation of the bones of the joint, so X-ray can still certainly provide insight into the disease progression.
|
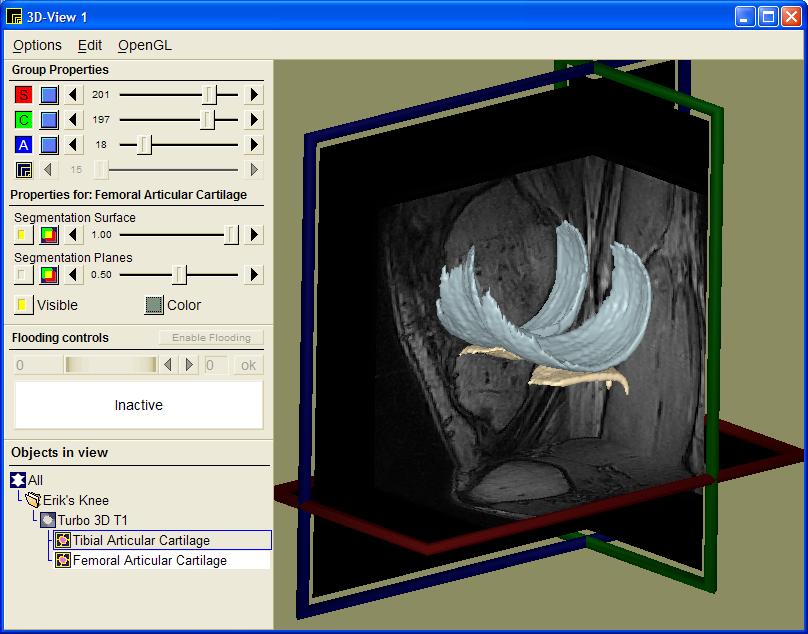
Our approach is to first model the entire articular cartilage from MR scans of the knee (see figure 3). From this 3D model of the cartilage, we have design specific measures that accurately describe the condition of the cartilage in terms of volume, area, thickness, congruity, smoothness, and homogeneity.
|
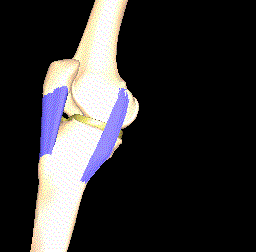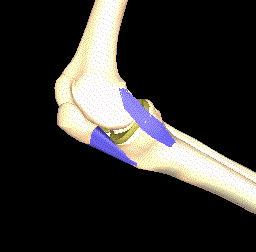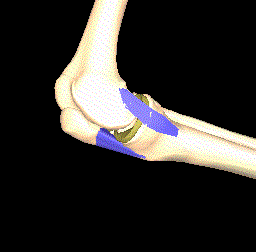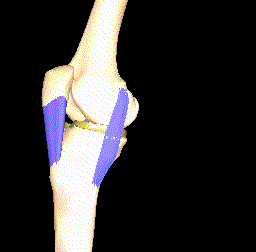
Another interesting approach is to model the kinematics of the knee joint (see Figure 4) since this affects the load distribution and thereby the importance of the local parts of articular cartilage. Thin cartilage is not necessarily a problem if it is located at a part of the bone that is never put under particular pressure.
|
The theoretical foundation for the projects mainly lies in supervised learning, shape modeling, shape statistics and geometry. We currently apply voxel classification and medial shape models to model the articular cartilage and intend to extend our models to the entire knee joint complex.
The work takes advantage of the present knowledge in the image group with regards to shape modelling as well as provides inspiration for further theoretical developments. For more information on shape modelling, shape statistics, and correspondence see Image group methodologies.
Main contact for the project at Nordic Bioscience is Erik Dam (http://www.diku.dk/~erikdam or erikdam@nordicbioscience.com). Other people involved at DIKU, CCBR, or Nordic Bioscience are Mads Nielsen, Marco Loog, Marleen de Bruijne, Morten Karsdal, Mikkel Fischer, Sudhakar Tummala, Paola Pettersen, Inger Byrjalsen, and Claus Christiansen.
We also collaborate closely with academic institutions in Denmark and abroad. At the Parker Institute at Frederiksberg Hospital, we collaborate mainly with Mikael Boesen and Henning Bliddal. At the Erasmus Medical Center, we collaborate with the MUSC group - in particular with Wiro Niessen, Albert Vossepoel, and Harrie Wienans.
CCBR has an Esaote C-span MR scanner that is used as the primary source for knee MR scans. In addition to that, CCBR and Nordic Bioscience has equipment for x-rays and analysis of serums and urine samples.
Students with interest in medical image analysis are welcome to inquire about possible projects. This goes for
A number of projects are possible in relation to the Osteo-Arthritis project. The OA project already has a collection of MRI scans and we are currently acquiring several other collections of knee scans. These data collections can be the focal point for a number of theoretically oriented projects:
In all the shape model related projects we will be inspired by the best components from Active Shape Models, Medial representations (m-rep), and Finite Element Models.
It is also possible to keep focus of the medical motivation for the project and define more application-oriented projects:
Especially for master students, the headlines above are probably too short to be really meaningful. Feel free to contact me at erikdam@nordicbioscience.com.